Induction
Induction is a fundamental concept in understanding long-range intermolecular interactions. It occurs when a molecule's electric field distorts the electronic distribution of a neighboring molecule, inducing a multipole moment in the latter. This induced multipole moment, in turn, interacts with the electric field of the inducing molecule, resulting in an attractive force between the two molecules.
What is polarizability?
In the context of molecular interactions, polarization plays a crucial role in understanding the behavior and properties of molecules and their response to electric fields. When an external electric field is applied to a molecule, it induces a distortion in the molecule's electron distribution, leading to the formation of induced multipole moments. This phenomenon is known as polarization.
Figure 1
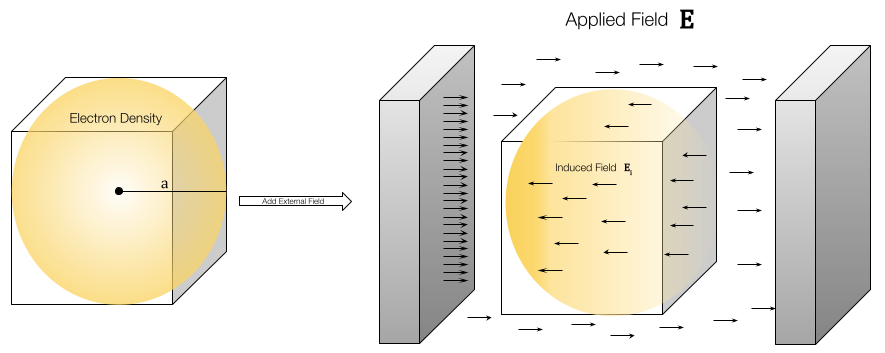
Imagine we have a spherical electron density distribution shown on the left. This negatively charge cloud can be attracted or repelled by shifting its density towards a positive source. We see this effect on the right where we apply a electric field with positive and negative charge on the left and right, respectively.
This is the result of a continuously applied electric field; with induction we instead are polarized by the electric field produced from multipole interactions. Thus, the response can fluctuate based on orientation and other quantum mechanical factors.
Electronic polarization occurs when the electron cloud of an atom within the molecule is displaced relative to the positive nucleus in the presence of an electric field. Polarizability is a measure of a molecule's ability to be polarized in response to an electric field. It is a molecular property that depends on factors such as the electronic structure, molecular geometry, and the strength of the applied field. Molecules with higher polarizability can undergo large shifts of their electron density and exhibit stronger polarization effects.
Induced dipole
Polarization has various effects on the behavior and properties of molecules. One important effect is the induction of dipole moments in neighboring molecules. When a molecule with a permanent dipole moment or an induced dipole moment interacts with a nearby molecule, it can induce a dipole moment in the latter. This induced dipole moment, in turn, interacts with the electric field of the inducing molecule, resulting in an attractive force between the two molecules. This interaction is known as the dipole-induced dipole interaction and contributes to the overall intermolecular forces.
Warning
You can induce high-order multipoles as well, but we will just consider dipoles here.
The induced dipole moment is defined as
where \(\alpha\) is the polarizability of the molecule and \(\mathcal{E}\) is external electric field.
TODO:
TODO: